Is salicylic acid polar or nonpolar – In the realm of chemistry, polarity plays a pivotal role in shaping the behavior of molecules. Salicylic acid, a widely used ingredient in skincare and pharmaceuticals, presents an intriguing case study for understanding the interplay between polarity and molecular properties.
This exploration delves into the molecular structure, functional groups, and chemical properties of salicylic acid to determine its polarity and its implications for its solubility, reactivity, and applications.
Salicylic acid, with its distinctive molecular structure, comprises a benzene ring adorned with a hydroxyl (-OH) group and a carboxylic acid (-COOH) group. These functional groups impart unique characteristics to the molecule, influencing its polarity and behavior in various solvents.
Salicylic Acid Properties
Salicylic acid, also known as 2-hydroxybenzoic acid, is a monohydroxybenzoic acid with the molecular formula C 7H 6O 3. It is a white, crystalline solid with a melting point of 159 °C and a boiling point of 270 °C. Salicylic acid is slightly soluble in water and more soluble in organic solvents such as ethanol and ether.
Molecular Structure
The molecular structure of salicylic acid consists of a benzene ring with a hydroxyl group (-OH) at the ortho position (adjacent to the carboxylic acid group -COOH). The carboxylic acid group is attached to the benzene ring at the meta position (opposite to the hydroxyl group).
Functional Groups, Is salicylic acid polar or nonpolar
Salicylic acid contains two functional groups: a hydroxyl group and a carboxylic acid group. The hydroxyl group is a polar functional group, while the carboxylic acid group is a weak acid. The presence of these functional groups gives salicylic acid its characteristic properties.
Chemical Properties
Salicylic acid is a weak acid with a pKa of 2.97. It is more acidic than benzoic acid but less acidic than acetic acid. Salicylic acid is also a good chelating agent, forming complexes with metal ions.
Polarity of Salicylic Acid
Polarity in Chemistry
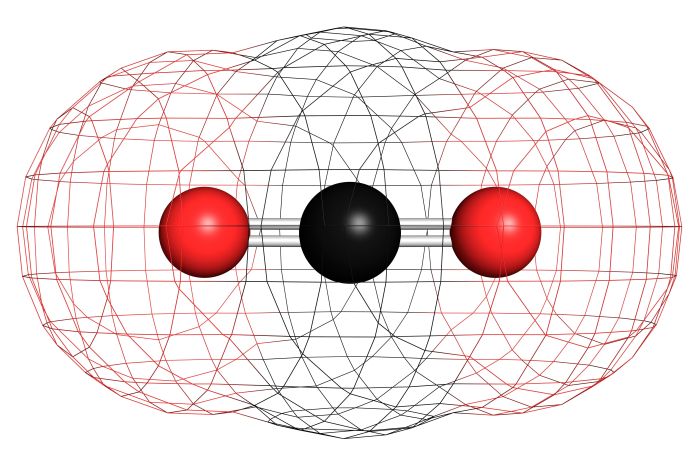
Polarity in chemistry refers to the uneven distribution of electrical charge within a molecule. A molecule is polar if it has a positive end and a negative end. The polarity of a molecule is determined by the electronegativity of its atoms and the molecular geometry.
Factors Contributing to Polarity
- Electronegativity: Electronegativity is the ability of an atom to attract electrons. The greater the difference in electronegativity between two atoms, the more polar the bond between them.
- Molecular Geometry: The molecular geometry of a molecule can also affect its polarity. A molecule with a symmetrical shape is less polar than a molecule with an asymmetrical shape.
Polarity of Salicylic Acid
Salicylic acid is a polar molecule due to the presence of the hydroxyl group and the carboxylic acid group. The hydroxyl group is more electronegative than the carbon atom it is bonded to, creating a partial negative charge on the oxygen atom.
The carboxylic acid group also has a partial negative charge on the oxygen atom. The two partial negative charges create a dipole moment, making salicylic acid a polar molecule.
Implications of Polarity: Is Salicylic Acid Polar Or Nonpolar
Solubility
The polarity of salicylic acid affects its solubility in different solvents. Salicylic acid is more soluble in polar solvents, such as water and ethanol, than in nonpolar solvents, such as hexane. This is because the polar functional groups of salicylic acid interact favorably with the polar molecules of the solvent.
Reactivity
The polarity of salicylic acid also affects its reactivity. Salicylic acid is more reactive with polar reagents, such as bases and oxidizing agents, than with nonpolar reagents. This is because the polar functional groups of salicylic acid are more likely to interact with the polar functional groups of the reagents.
Applications
The polarity of salicylic acid influences its applications. Salicylic acid is used as a topical medication for the treatment of acne and psoriasis. It is also used as a preservative in food and cosmetics. The polarity of salicylic acid allows it to penetrate the skin and interact with the underlying tissues.
Comparison with Other Compounds
Table
| Compound | Molecular Structure | Functional Groups | Polarity |
|---|---|---|---|
| Salicylic acid | C7H6O3 | Hydroxyl group, carboxylic acid group | Polar |
| Benzoic acid | C7H6O2 | Carboxylic acid group | Nonpolar |
| Aspirin | C9H8O4 | Carboxylic acid group, acetyl group | Nonpolar |
Similarities and Differences
- Salicylic acid, benzoic acid, and aspirin are all aromatic compounds with a benzene ring.
- Salicylic acid and benzoic acid both have a carboxylic acid group, but salicylic acid also has a hydroxyl group.
- Aspirin has a carboxylic acid group and an acetyl group.
- Salicylic acid is polar, while benzoic acid and aspirin are nonpolar.
Implications of Polarity
The polarity of these compounds affects their properties and applications. Salicylic acid is more soluble in water than benzoic acid or aspirin. Salicylic acid is also more reactive with polar reagents than benzoic acid or aspirin. Benzoic acid and aspirin are more volatile than salicylic acid.
Questions Often Asked
Is salicylic acid a polar or nonpolar molecule?
Salicylic acid is a polar molecule due to the presence of both polar (-OH) and nonpolar (-COOH) functional groups.
How does polarity affect the solubility of salicylic acid?
Polarity influences the solubility of salicylic acid in different solvents. It is more soluble in polar solvents like water and less soluble in nonpolar solvents like oil.
What are some applications of salicylic acid based on its polarity?
Salicylic acid’s polarity makes it suitable for use in skincare products as it can penetrate the skin’s oily layers and in pharmaceutical formulations as it can interact with polar biological molecules.