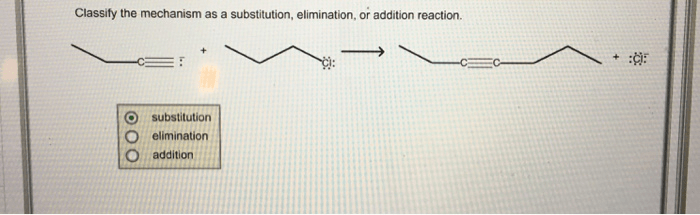
Classify the mechanism as a substitution elimination or addition reaction – Chemical reactions can be classified into three main types: substitution, elimination, and addition. These classifications are based on the changes that occur to the molecular structure of the reactants during the reaction. Substitution reactions involve the replacement of one atom or group of atoms with another, while elimination reactions involve the removal of atoms or groups of atoms from a molecule.
Addition reactions, on the other hand, involve the addition of atoms or groups of atoms to a molecule.
Understanding the mechanisms of these reactions is crucial for comprehending the behavior of chemical compounds and predicting the outcome of chemical reactions.
Substitution Reaction
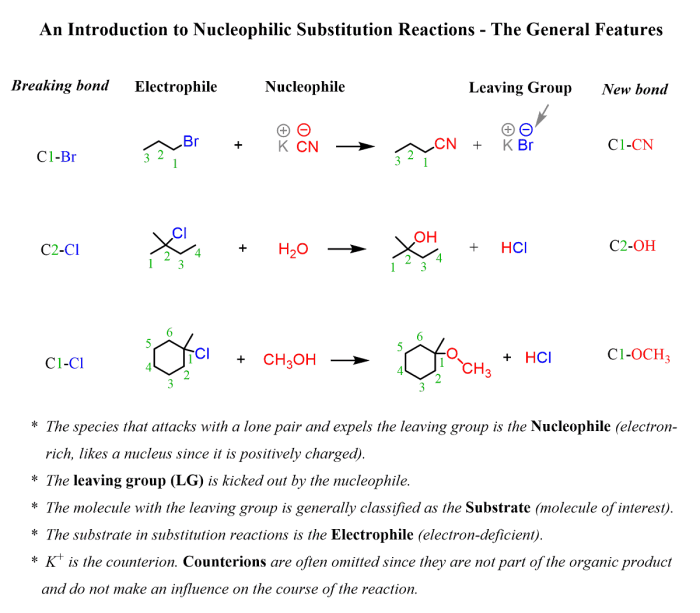
Substitution reactions involve the replacement of one atom or group of atoms in a molecule with another atom or group of atoms. They are typically classified as nucleophilic substitution or electrophilic substitution, depending on the nature of the attacking species.Nucleophilic
substitution reactions occur when a nucleophile (a species with a lone pair of electrons) attacks an electrophile (a species with a positive charge or an electron-deficient atom). The nucleophile donates its lone pair of electrons to the electrophile, forming a new bond and displacing the leaving group.
Electrophilic substitution reactions occur when an electrophile attacks a nucleophile, forming a new bond and displacing the leaving group.
Examples of Substitution Reactions, Classify the mechanism as a substitution elimination or addition reaction
- SN2 reaction: A nucleophile attacks a primary or secondary alkyl halide, resulting in inversion of configuration at the carbon atom.
- SN1 reaction: A nucleophile attacks a tertiary alkyl halide, resulting in racemization at the carbon atom.
- Electrophilic aromatic substitution: An electrophile attacks an aromatic ring, resulting in the substitution of a hydrogen atom with the electrophile.
Elimination Reaction
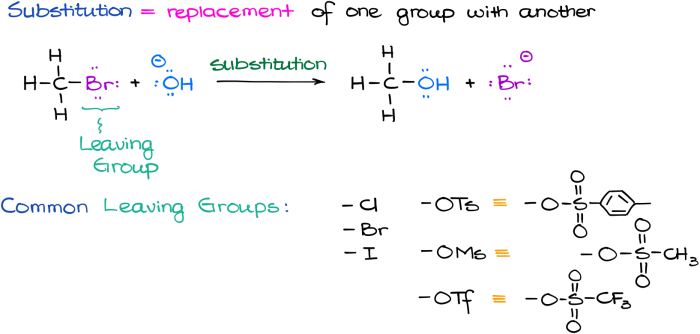
Elimination reactions involve the removal of two atoms or groups of atoms from a molecule, resulting in the formation of a double or triple bond. They are typically classified as E2 or E1 reactions, depending on the mechanism of the reaction.E2
reactions occur in a concerted manner, meaning that the two bonds break and the new double bond forms simultaneously. E1 reactions occur in a stepwise manner, meaning that one bond breaks first, forming a carbocation intermediate, which then reacts with a base to form the double bond.
Examples of Elimination Reactions
- E2 reaction: A strong base abstracts a proton from a carbon atom adjacent to a leaving group, resulting in the formation of a double bond.
- E1 reaction: A weak base abstracts a proton from a carbon atom adjacent to a leaving group, resulting in the formation of a carbocation intermediate, which then reacts with a base to form the double bond.
Addition Reaction
Addition reactions involve the addition of one or more atoms or groups of atoms to a molecule, resulting in the formation of a new bond. They are typically classified as electrophilic addition or nucleophilic addition, depending on the nature of the attacking species.Electrophilic
addition reactions occur when an electrophile attacks a nucleophile, forming a new bond and displacing the leaving group. Nucleophilic addition reactions occur when a nucleophile attacks an electrophile, forming a new bond and displacing the leaving group.
Examples of Addition Reactions
- Electrophilic addition of hydrogen: Hydrogen adds to a double bond, resulting in the formation of a saturated alkane.
- Nucleophilic addition of water: Water adds to a carbonyl group, resulting in the formation of a gem-diol.
Classification of Reactions
Reactions can be classified as substitution, elimination, or addition reactions based on the following criteria:
- Substitution reactionsinvolve the replacement of one atom or group of atoms with another.
- Elimination reactionsinvolve the removal of two atoms or groups of atoms, resulting in the formation of a double or triple bond.
- Addition reactionsinvolve the addition of one or more atoms or groups of atoms to a molecule, resulting in the formation of a new bond.
The following table summarizes the key characteristics of each type of reaction:
| Reaction Type | Mechanism | Examples |
|---|---|---|
| Substitution | Nucleophilic or electrophilic attack | SN2, SN1, electrophilic aromatic substitution |
| Elimination | E2 or E1 mechanism | E2, E1 |
| Addition | Electrophilic or nucleophilic attack | Electrophilic addition of hydrogen, nucleophilic addition of water |
Classifying reactions is important because it allows us to understand the mechanism of the reaction and predict the products that will be formed.
FAQ: Classify The Mechanism As A Substitution Elimination Or Addition Reaction
What are the key characteristics of substitution reactions?
Substitution reactions involve the replacement of one atom or group of atoms with another. They typically occur when a nucleophile (an electron-rich species) attacks an electrophile (an electron-deficient species).
What are some examples of elimination reactions?
Elimination reactions involve the removal of atoms or groups of atoms from a molecule. A common example is the E2 elimination reaction, which involves the removal of a proton and a leaving group from a molecule.
How can I identify addition reactions?
Addition reactions involve the addition of atoms or groups of atoms to a molecule. They typically occur when an electrophile attacks a nucleophile.