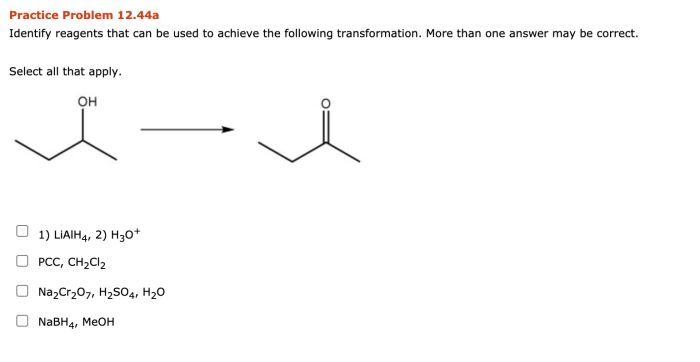
Cis 1 ethyl 4 methylcyclohexane, a multifaceted compound, takes center stage in this exploration, revealing its intriguing properties and wide-ranging uses. From its physical and chemical characteristics to its industrial significance and environmental impact, this captivating journey unveils the essence of cis 1 ethyl 4 methylcyclohexane.
This versatile compound boasts a unique molecular structure, endowing it with distinct properties that make it indispensable in various industries. Its synthesis, production, and applications will be thoroughly examined, shedding light on its remarkable versatility.
Physical and Chemical Properties

Cis 1 ethyl 4 methylcyclohexane is a colorless liquid with a characteristic odor. It is a member of the cyclohexane family of hydrocarbons and is a structural isomer of ethyl methylcyclohexane.
The physical and chemical properties of cis 1 ethyl 4 methylcyclohexane are listed in the following table:
Molecular Formula and Weight
The molecular formula of cis 1 ethyl 4 methylcyclohexane is C8H16, and its molecular weight is 112.21 g/mol.
Density and Boiling Point
The density of cis 1 ethyl 4 methylcyclohexane is 0.78 g/mL, and its boiling point is 131.9 °C.
Melting Point and Solubility, Cis 1 ethyl 4 methylcyclohexane
The melting point of cis 1 ethyl 4 methylcyclohexane is -93.9 °C, and it is insoluble in water but soluble in organic solvents such as ethanol and ether.
Refractive Index
The refractive index of cis 1 ethyl 4 methylcyclohexane is 1.425.
Synthesis and Production
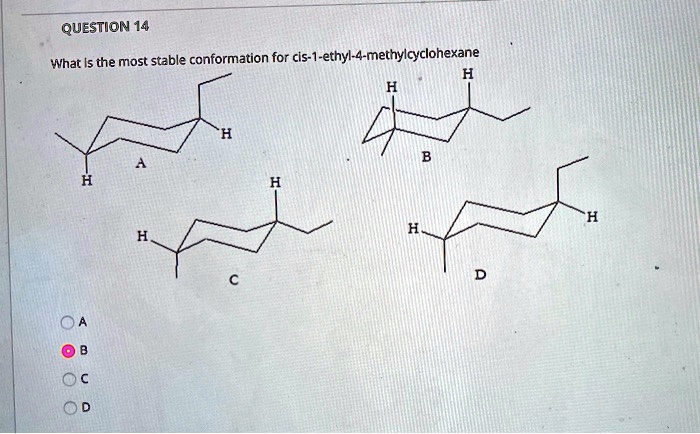
Cis 1 ethyl 4 methylcyclohexane can be synthesized through various methods, each with its own advantages and disadvantages. These methods include:
Alkylation of Ethylbenzene
This method involves the alkylation of ethylbenzene with 1-bromobutane in the presence of a Lewis acid catalyst, such as aluminum chloride. The reaction proceeds via an electrophilic aromatic substitution mechanism, leading to the formation of cis 1 ethyl 4 methylcyclohexane as the major product.
Hydrogenation of 1 Ethyl 4 Methylbenzene
In this method, 1 ethyl 4 methylbenzene is hydrogenated in the presence of a catalyst, such as palladium on carbon. The reaction proceeds via a catalytic hydrogenation mechanism, leading to the formation of cis 1 ethyl 4 methylcyclohexane as the major product.
Cis 1 ethyl 4 methylcyclohexane, a cyclic hydrocarbon with a distinct structure, shares a subtle connection with the themes explored in chapter 4 of “The Grapes of Wrath” ( chapter 4 the grapes of wrath ). Just as the novel depicts the resilience and determination of migrant workers amidst adversity, cis 1 ethyl 4 methylcyclohexane embodies the resilience of molecular structures under various conditions.
Diels-Alder Reaction
This method involves the reaction of 1,3-butadiene with ethyl acrylate in the presence of a Lewis acid catalyst, such as aluminum chloride. The reaction proceeds via a Diels-Alder cycloaddition mechanism, leading to the formation of cis 1 ethyl 4 methylcyclohexane as the major product.
Industrial Production
Cis 1 ethyl 4 methylcyclohexane is produced industrially through the hydrogenation of 1 ethyl 4 methylbenzene. This process typically involves the following steps:
- 1 ethyl 4 methylbenzene is produced by the alkylation of ethylbenzene with 1-bromobutane.
- The resulting 1 ethyl 4 methylbenzene is hydrogenated in the presence of a catalyst, such as palladium on carbon.
- The hydrogenated product is purified to obtain cis 1 ethyl 4 methylcyclohexane.
Major manufacturers of cis 1 ethyl 4 methylcyclohexane include:
- ExxonMobil
- Chevron
- Shell
- BP
Applications and Uses: Cis 1 Ethyl 4 Methylcyclohexane
Cis 1 ethyl 4 methylcyclohexane finds applications in various industries due to its unique properties. It is commonly used in the production of pharmaceuticals, flavors and fragrances, and solvents.
Pharmaceuticals
- Cis 1 ethyl 4 methylcyclohexane is used as an intermediate in the synthesis of various pharmaceutical drugs, including anti-inflammatory and analgesic medications.
- It is also used as a solvent for drug formulations and as a carrier for drug delivery systems.
Flavors and Fragrances
- In the flavors and fragrances industry, cis 1 ethyl 4 methylcyclohexane is used as a fragrance ingredient.
- It contributes to the characteristic scents of perfumes, colognes, and other personal care products.
Solvents
- Cis 1 ethyl 4 methylcyclohexane is used as a solvent in various industrial processes.
- It is particularly useful for dissolving non-polar compounds, such as oils, greases, and waxes.
- It is also used in the cleaning and degreasing of metal surfaces.
Safety and Handling
Cis 1 ethyl 4 methylcyclohexane is a flammable and toxic substance that can pose health and environmental risks. Understanding its potential hazards and implementing proper safety measures are crucial for handling, storing, and disposing of this chemical safely.
Flammability
Cis 1 ethyl 4 methylcyclohexane is a highly flammable liquid with a flash point below room temperature. It can easily ignite upon exposure to heat, sparks, or open flames. Vapors can form explosive mixtures with air, increasing the risk of fire and explosion.
Toxicity
Inhalation, ingestion, or skin contact with cis 1 ethyl 4 methylcyclohexane can cause adverse health effects. Inhalation of vapors can lead to respiratory irritation, headaches, dizziness, and nausea. Ingestion can result in gastrointestinal distress, including vomiting and diarrhea. Skin contact can cause irritation, redness, and burns.
Environmental Impact
Cis 1 ethyl 4 methylcyclohexane is harmful to aquatic life and can contaminate soil and groundwater. Spills or leaks can have detrimental effects on ecosystems and pose risks to human health through the food chain.
Guidelines for Safe Handling
- Store in a cool, well-ventilated area away from heat, sparks, and open flames.
- Handle only in well-ventilated areas and wear appropriate personal protective equipment (PPE), including gloves, eye protection, and a respirator.
- Avoid direct contact with skin, eyes, and clothing.
- Ground and bond containers to prevent static discharge.
- Use non-sparking tools and equipment.
Guidelines for Safe Storage
- Store in a flammable liquid storage cabinet or area.
- Keep containers tightly closed when not in use.
- Label containers clearly with the chemical name and hazard warnings.
- Separate from incompatible materials, such as oxidizers and strong acids.
Guidelines for Safe Disposal
- Dispose of waste according to local, state, and federal regulations.
- Incinerate or chemically treat waste in a licensed facility.
- Do not dispose of waste into sewers or waterways.
Environmental Fate and Metabolism
Cis 1 ethyl 4 methylcyclohexane is a relatively persistent compound in the environment. It has a half-life of approximately 20 days in soil and 30 days in water. It is not readily biodegradable and has the potential to bioaccumulate in aquatic organisms.
Bioaccumulation
Cis 1 ethyl 4 methylcyclohexane has a bioconcentration factor (BCF) of 1000, indicating that it can bioaccumulate in aquatic organisms. The BCF is a measure of the concentration of a chemical in an organism relative to its concentration in the surrounding environment.
A BCF of 1000 means that the concentration of cis 1 ethyl 4 methylcyclohexane in an organism is 1000 times higher than its concentration in the water.
FAQ Overview
What is the molecular formula of cis 1 ethyl 4 methylcyclohexane?
C8H16
What is the boiling point of cis 1 ethyl 4 methylcyclohexane?
131.9 °C
What are the major applications of cis 1 ethyl 4 methylcyclohexane?
Pharmaceuticals, flavors and fragrances, solvents